Crimped Woven Mesh Material :
High carbon steel:45#,50#,55#,60#,70#,65Mn,72A,Stainless steel 304,321,316,2205,2507
Wire diameter: 1.37mm-12.7mm,Our finished wire is inspected by third party SGS,
Tolerance+_0.03mm .
Aperture/Opening:from 2mm to 100mm,tolerance +-3%
1.Aperture error:<3%,which is better than international standards ;
2.Wire diameter error :+-3%;
3.Diagonal error:<9mm every 2m .
Weaving Types :double crimped
screen,single intermediate crimp screen,double intermediate crimp
screen,lock crimp screen,flat top screen,pressure welded screen .
As
a highly-accomplished wire mesh exporter, Kingdelong was established
jointly by China and South Korea in 1986, Our strategic location in
Anping County,Hebei province is only 300KM away from Beijing and
Tianjin, which offers us very competitive advantages both in trade and
logistics. Through two decades of hard work, Kingdelong company had
evolved into one of China`s largest wire mesh manufacturing
companies,Covering an area of over 200,000㎡, certificated by ISO 9001
& ISO 14001,as well as more than 450 diversified production
machines, which are either imported from foreign countries or developed
in-house.Wire mesh products find applications in a wide range of
industries, from Pharmacy to petroleum and chemical to textile. Decorative Metal Mesh,Metal Mesh Curtain,Decorative Ring Mesh,Flexible Metal Mesh Curtain ANPING KINDELONG WIRE MESH CO.,LTD. , https://www.apkdlwiremesh.com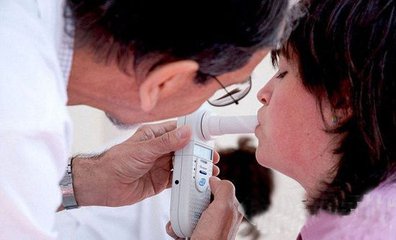
US company obtains preliminary results with mass spectrometry for detection of respiratory diseases in exhaled air
China Instrument Network Instrument R&D Recently, 1st Detect, a subsidiary of NASDAQ-listed Astrotech (ASTC), announced that the use of mass spectrometry for exhaled breath analysis in humans can be applied to the diagnosis and treatment of respiratory diseases. The preliminary results of clinical trials have been obtained.
Image from the network
1st Detect, in collaboration with the University of Texas Health Science Center (now UT Health San Antonio), announced preliminary results of the BreathDetect 1000 preclinical trial, a rapid bedside ventilator for detecting respiratory bacterial infections, such as Acquired pneumonia (HAP).
Dr. Edward G Brooks of San Antonio and his team aimed at the two most deadly bacterial strains found in HAP. Validation after the bacterial culture headspace test is currently in progress. The first human trial started on May 22, 2017.
Brooks said: "The first test of BreathDetect 1000 has successfully identified several unique volatile organic compound (VOC) metabolites from Staphylococcus and Pseudomonas in human breath samples. BreathDetect 1000 can significantly improve physicians' correct treatment Patient's ability. By sampling directly from the lungs, BreathDetect 1000 can greatly speed up the current diagnostic process, which in turn can reduce the misdiagnosis of the disease and subsequent antibiotic resistance, reduce hospital stay, and most importantly save lives."
If new technologies are not used, doctors now have to wait three days to determine the organisms through the laboratory and antibiotic resistance analysis for another two days. At the same time, they developed broad-spectrum antibiotics, which led to an urgent public health threat of antibiotic resistance and led to the recurrence of a fatal disease such as methicillin-resistant Staphylococcus aureus (MRSA).
"In the early stages of product validation, we highly encouraged the high specificity of the BreathDetect 1000 and UT Health San Antonio's progress in obtaining and validating human breath samples." 1st Detect CEO Thomas B. Pickens III said, "And the parent company Astrotech Extra Human testing and development are ongoing."
(Original title: US company used mass spectrometry to detect exhaled air diagnosis and treatment of respiratory diseases has entered clinical trials)