Combination Lathe Milling Machine Combination Lathe Milling Machine has the function of turning, milling, drilling and thread cutting. Feed can be controlled automatically or manually, suitable for processing metal, wood and other materials. It is extensively used in job-shops, teaching, scientific research, occupation training, especially in house for household utensils.The machine has the character of compact construction, easy operating and wide-range speed. The function of turning, drilling, and milling can be made in one machine. Worktable feed can be controlled automatically or manually in longitudinal and cross direction. The drilling -milling headstock can be rotated 180°. The machine was designed according to CE standard. It also can be controlled by personal computer when connecting with it. It meets all requirements of precision mechanics, training workshops and education. The machining centre allows drilling, milling and turning on minimum space, comes with a digital display of drill depth and speed for high comfort and features a large spindle bore. Combination Lathe Milling Machine,Lathe Milling Machine,Combination Lathe Machine,Mini Lathe Milling Machine Tengzhou Borui CNC Machine Tool Co,.Ltd , https://www.br-cnc.com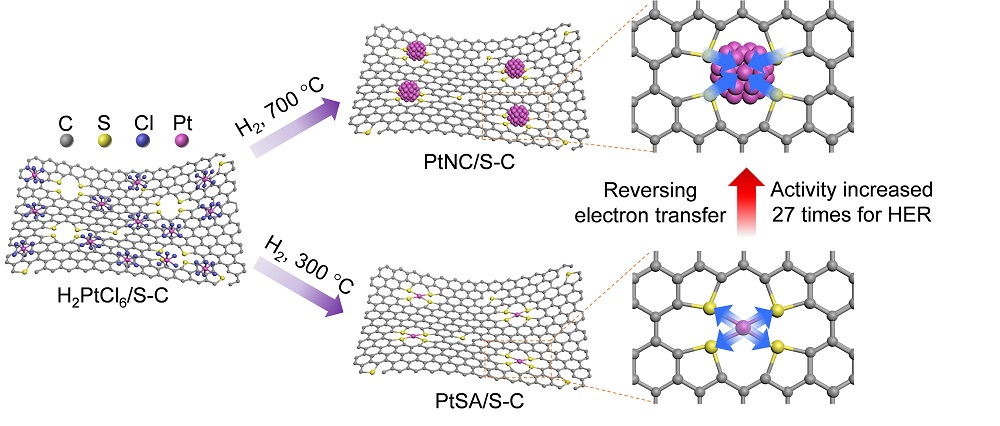
China University of Science and Technology Progress in the Study of Catalyst Metal-Carrier Strong Interaction
[ Instrument Network Instrument Development ] Loaded metal catalysts are critical to modern industry. A large number of experimental and theoretical studies have shown that the carrier in the supported metal catalyst not only plays a role in dispersing and stabilizing the metal nanoparticles, but also strongly interacts with the metal particles, thereby affecting the activity, selectivity and stability of the catalyst. Recently, Professor Liang Haiwei of the University of Science and Technology of China and Wu Xiaojun's research group conducted a joint research on experiments and theories. Based on the sulfur-doped carbon-supported platinum (Pt) catalyst system, it was found that the direction of charge transfer between the metal and the carbon carrier A new phenomenon of reversal of changes in Pt size (Figure 1). The research results were published in the international journal Nature Communications under the title Reversing the charge transfer between platinum and sulfur-doped carbon support for electrocatalytic hydrogen evolution. The co-first authors of the paper are Ph.D. students Yan Qiangqiang and Wu Daoxiong.
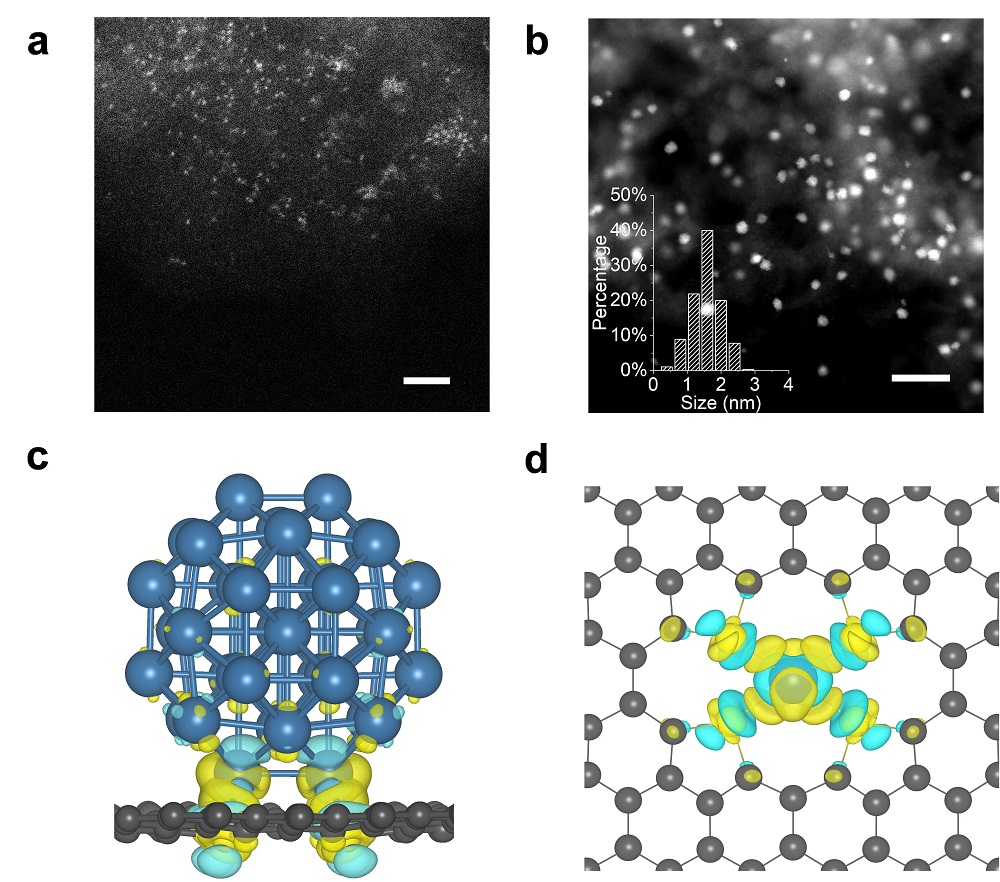
In this work, the researchers first synthesized sulfur-doped mesoporous carbon using their pre-developed transition metal catalyzed methods of carbonizing organic small molecules (Nature Communications 2015, 6, 7992; Science Advances 2018, 4, eaat0788). SC) A carrier material and two different Pt catalyst materials were prepared using the SC support material by conventional impregnation: Pt monoatomic (PtSA/SC) and Pt nanoclusters (PtNC/SC) catalysts. Characterization of spherical aberration-corrected transmission electron microscopy (HAADF-STEM) and synchrotron radiation X-ray absorption spectra (EXAFS and XANES) confirmed the structural characteristics of Pt monoatomic and Pt nanoclusters, ie both directly with sulfur atoms on carbon supports. Bond. Interestingly, by analyzing the X-ray photoelectron spectroscopy (XPS) and XANES spectra of the comparative samples, the researchers found a new phenomenon in which the direction of charge transfer between the Pt and the SC carrier reversed with the change in Pt size: When Pt is a single atom, its electrons are transferred to the SC carrier; and when Pt is a 1.5 nm cluster, electrons are transferred from the SC carrier to Pt. At the same time, the theoretically calculated charge analysis results further confirm the reversal of charge transfer between the Pt and SC carriers.
Further, the researchers explored the electrocatalytic hydrogen evolution reaction performance of the above two catalysts. The experimental results show that the catalytic activity of Pt nanoclusters with electron-rich properties is significantly better than that of electron-deficient Pt monoatoms and commercial Pt/C catalysts. Theoretical calculations show that for the Pt cluster system, the electrons on the SC carrier are transferred to the Pt cluster, resulting in an increase in the electron cloud density on the Pt, a decrease in the Gibbs free energy of the H* adsorption, and an increase in the HER activity. This work describes a new understanding of the charge transfer phenomenon between metals and carriers, and provides a new idea based on charge transfer regulation for efficient catalyst design.
The research was funded by the National Natural Science Foundation of China, the National Key Research and Development Program Fund, the Fundamental Research Fund for Central Universities, and the China University of Science and Technology Synchrotron Radiation Joint Fund.